Research, Technology and IP
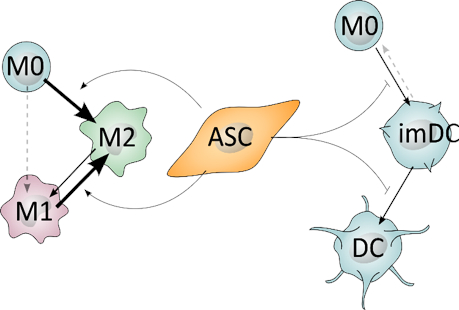
ASCs are capable of performing anti-inflammatory action. They can affect a variety of immune cells from inflammatory to anti-inflammatory cell types. In this example ASCs inhibit differentiation of monocytes (M0) into inflammatory macrophages (M1), while facilitating differentiation of M0 and M1 into the anti-inflammatory macrophage (M2). In addition, ASCs inhibit M0 differentiation immature dendritic cells and subsequently mature dendritic cells (imDC and DC, respectively). These immune cell types are key components in many diseases. Figure by Morten Juhl, PhD.
STEM CELLS
Mesenchymal stromal cells are nonspecific and undifferentiated stem cells that can play an essential role in tissue regeneration and have strong immunomodulatory properties.
Stem cell therapy is a promising new therapeutic concept. Pre-clinical studies and clinical trials suggest that stem cells can be used in the treatment of multiple disease conditions with involvement of inflammation and immunological activity, blood circulatory disturbance, fibrosis and scar development.
Stem cells have primarily been applied in personalized disease treatment settings where each patient is the source of the stem cells used. Generating clinically relevant and efficient numbers of stem cells for therapeutic use from each individual patient is however challenging. Effective dissemination and use of stem cell treatments require robust, effective, high yield manufacturing, storage and distribution technologies. Cell2Cure solves this by sourcing cells form healthy donors and by using industrially scalable manufacturing technology.
PRODUCT AND IP
The ASC products are Advanced Therapy Investigational Medicinal Products (ATIMP) isolated and manufactured from adipose tissue from healthy donors. The cell products are stored in nitrogen vapour tanks as “off-the-shelf” products ready to use after 5-10 minutes of thawing. Therefore, products are easily disseminated and readily be administered to any patient in need.
Mode of Action
Interactions between the ASCs and the diseased tissue induces release of a wide range of factors secreted from the ASCs and the adjacent tissue. This simultaneous activation of multiple regenerative pathways initiates tissue regeneration processes such as reduced inflammation, growth of new blood vessels, and reduced fibrosis and scar formation.
MANUFACTURE
The ASC products are allogeneic cryopreserved ready-to-use pharmaceutical products, manufactured according to GMP and all regulatory requirements.
Cell2Cure Manufacturing involves expansion of the cells without any animal derived components in automated closed bioreactor systems within a controlled cell-culture environment.
Production is highly scalable and each production batch (based on a single donation of cells) results in the manufacture of 50 – 200 treatment doses depending on dosage size needed.
Several dosages and formulations are being manufactured suitable for different disease indications.
Cell2Cure has entered into a collaboration agreement with Cbio A/S to establish C2C´s own GMP facility for production in Cbio´s approved cell production facility in Søborg, Denmark.
SAFETY
More than 300 patients have been treated with the ASC products without any safety concerns related to the products in patients with heart failure, left bundle branch block in the heart, reduced tear production in Sjögren’s syndrome, radiation-induced hyposalivation and xerostomia in previous head and neck cancer patients and in lung transplantation patients.
Our future IP strategy will aim to expand and strengthen IP around the ASC technology, based on new patents within several disease indications – in the areas of production, storage and distribution of adipose tissue derived mesenchymal stromal cells.
PATENT
The Cell2Cure core technology is patent protected to secure C2C key asset. C2C has patented the technological concept for industrial production, upscaling, and distribution of ASC products.
“STEM CELL THERAPY BASED ON ADIPOSE-DERIVED STEM CELLS”
Europe: Patent number EP3365432B1 / WO 2017-068140. Priority: October 23. 2015.
Australia: Patent number AU2016342387A1. Priority: October 23. 2015
USA: Pub. No.: US 2018/0325957 A1; Pub. Date: Nov. 15, 2018, Application number: 15/769,873
The patent is issued in Australia and the 19 European PCT countries (Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, United Kingdom, Ireland, Italy, Luxembourg, Netherlands, Norway, Poland, Portugal, Sweden, Slovenia and Turkey). Furthermore, the patent is filed and pending in Canada, China, Hong Kong, Japan, The Republic of Korea and in USA (Application 15/769,873).
CONTACT US

Innobooster grant

SME Instrument grant
This project has received funding from the European Union’s SME Instrument, Horizon 2020 research and innovation programme under grant agreement No [H2020-SMEINST-868284_PRO-SCT]

Grand Solution Grant
Cell2Cure, as part of the consortium Clinical Stem cell Innovation and production Center, CSIC, receives funding from Innovation Fund Denmark.